Diffuse Optical Tomography Systems in 2025: Unveiling the Next Wave of Non-Invasive Imaging Innovation. Explore Market Acceleration, Technological Advances, and Strategic Opportunities Shaping the Future.
- Executive Summary: Key Findings and Market Highlights
- Market Size and Growth Forecast (2025–2029): Trends and Projections
- Technology Landscape: Innovations in Diffuse Optical Tomography
- Competitive Analysis: Leading Companies and Strategic Moves
- Clinical Applications and Expanding Use Cases
- Regulatory Environment and Industry Standards
- Regional Market Dynamics: North America, Europe, Asia-Pacific, and Beyond
- Investment, M&A, and Funding Trends
- Challenges, Barriers, and Risk Factors
- Future Outlook: Opportunities and Strategic Recommendations
- Sources & References
Executive Summary: Key Findings and Market Highlights
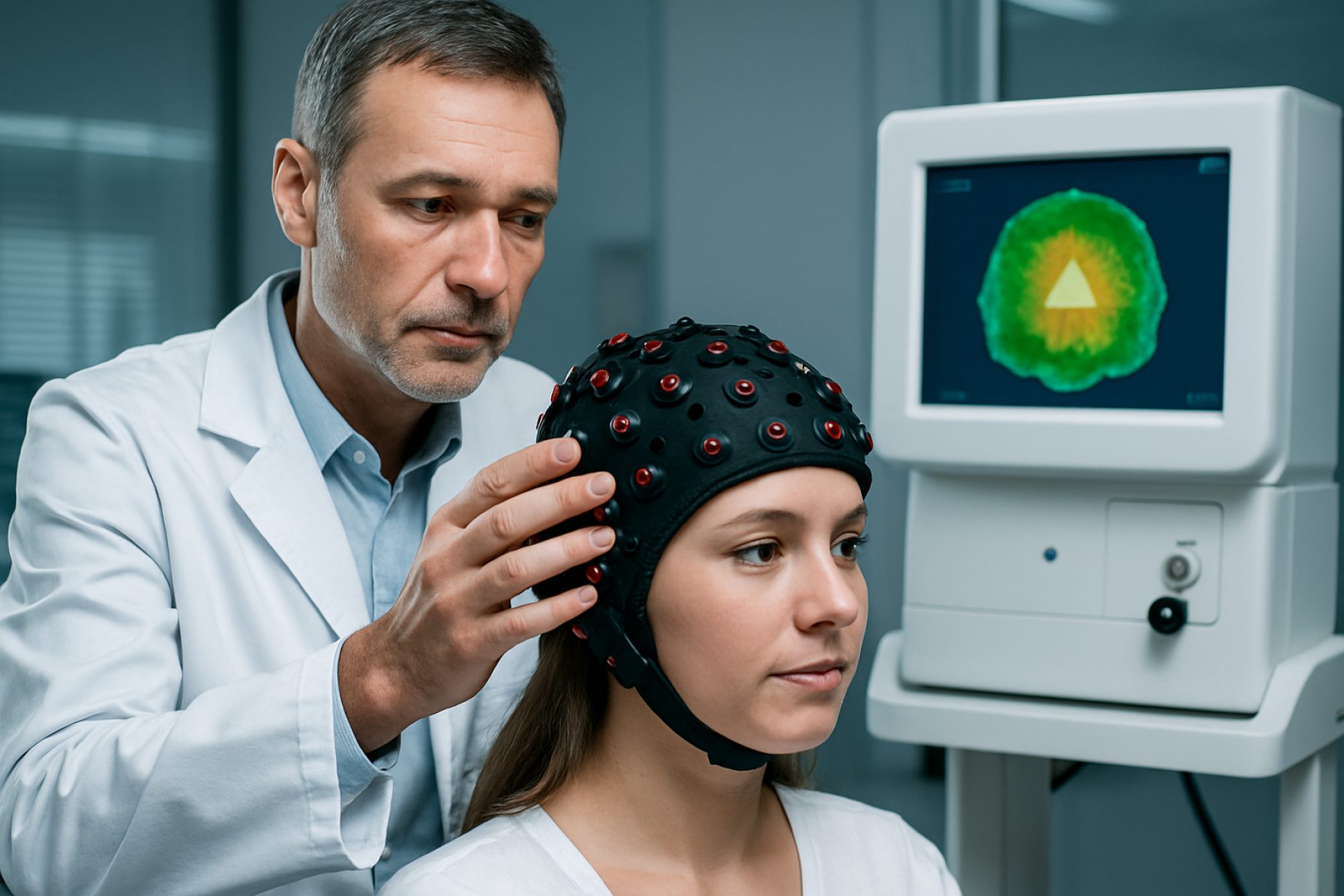
Diffuse Optical Tomography (DOT) systems are emerging as a pivotal imaging modality in biomedical research and clinical diagnostics, leveraging near-infrared light to generate functional and structural images of biological tissues. As of 2025, the DOT market is characterized by robust innovation, expanding clinical applications, and increasing adoption in both academic and healthcare settings. Key findings and market highlights for this sector are summarized below.
- Technological Advancements: Recent years have seen significant improvements in DOT system sensitivity, spatial resolution, and portability. Leading manufacturers such as Hamamatsu Photonics and Artinis Medical Systems have introduced next-generation devices with enhanced multi-channel capabilities and real-time imaging, supporting both research and bedside clinical use.
- Expanding Clinical Applications: DOT is increasingly utilized for brain imaging, breast cancer detection, and neonatal monitoring. The non-invasive, radiation-free nature of DOT makes it particularly attractive for pediatric and longitudinal studies. Companies like NeuroMetrix and NIRx Medical Technologies are actively developing systems tailored for neurological and cognitive research, reflecting a trend toward specialized, application-driven solutions.
- Integration with Other Modalities: There is a growing trend toward hybrid imaging systems that combine DOT with modalities such as MRI or ultrasound, aiming to provide complementary anatomical and functional information. This integration is expected to enhance diagnostic accuracy and broaden the clinical utility of DOT systems in the coming years.
- Market Drivers and Adoption: The demand for non-invasive, cost-effective imaging solutions is fueling DOT adoption, particularly in regions with expanding healthcare infrastructure. Academic research institutions and hospitals are key end-users, with increased funding for neuroimaging and oncology research further propelling market growth.
- Outlook: The DOT market is poised for continued expansion through 2025 and beyond, driven by ongoing technological innovation, broader clinical validation, and strategic collaborations between device manufacturers and research organizations. As regulatory pathways become clearer and reimbursement frameworks evolve, DOT systems are expected to gain a stronger foothold in routine clinical practice.
In summary, the DOT sector in 2025 is marked by rapid technological progress, diversification of clinical applications, and a favorable outlook for sustained growth, with industry leaders such as Hamamatsu Photonics, Artinis Medical Systems, and NIRx Medical Technologies at the forefront of innovation and commercialization.
Market Size and Growth Forecast (2025–2029): Trends and Projections
The global market for Diffuse Optical Tomography (DOT) systems is poised for significant growth between 2025 and 2029, driven by technological advancements, expanding clinical applications, and increasing demand for non-invasive imaging modalities. DOT systems, which utilize near-infrared light to generate functional images of tissue, are gaining traction in neurology, oncology, and neonatal care due to their safety, portability, and ability to provide real-time physiological data.
Key industry players are investing in research and development to enhance system sensitivity, spatial resolution, and user-friendliness. Companies such as Hamamatsu Photonics and Hitachi are recognized for their advanced photonic and optical technologies, which underpin many DOT platforms. Artinis Medical Systems is another notable manufacturer, specializing in portable and wearable DOT and near-infrared spectroscopy (NIRS) devices, with a focus on brain and muscle imaging. NeuroLight and NIRx Medical Technologies are also active in the sector, offering systems for both research and clinical use.
Market expansion is further supported by the growing adoption of DOT in brain function monitoring, particularly in neonatal intensive care units and cognitive neuroscience research. The integration of DOT with other imaging modalities, such as MRI and EEG, is expected to broaden its clinical utility and drive demand. Additionally, the miniaturization of components and the development of wireless, wearable DOT systems are anticipated to open new avenues in ambulatory and home-based monitoring.
From 2025 onward, the market is projected to experience a compound annual growth rate (CAGR) in the high single digits, reflecting both increased healthcare spending and the rising prevalence of neurological and oncological disorders. North America and Europe are expected to remain leading regions due to robust research infrastructure and early adoption of innovative medical technologies. However, Asia-Pacific is forecasted to witness the fastest growth, propelled by expanding healthcare access and investments in medical device innovation, particularly in countries like China and Japan.
Looking ahead, the DOT systems market is likely to benefit from ongoing collaborations between device manufacturers, academic institutions, and healthcare providers. Regulatory approvals and standardization efforts will play a crucial role in accelerating clinical adoption. As companies such as Hamamatsu Photonics, Hitachi, and Artinis Medical Systems continue to innovate, the outlook for DOT systems remains robust, with expanding applications and a growing user base expected through 2029.
Technology Landscape: Innovations in Diffuse Optical Tomography
Diffuse Optical Tomography (DOT) systems are experiencing a period of rapid technological advancement, driven by the convergence of photonics, advanced detectors, and computational imaging. As of 2025, DOT is increasingly recognized for its non-invasive, radiation-free imaging capabilities, particularly in functional brain imaging, breast cancer detection, and neonatal care. The technology landscape is shaped by both established medical device manufacturers and innovative startups, each contributing to the evolution of system performance, portability, and clinical applicability.
Key players in the DOT sector include Hamamatsu Photonics, a global leader in photonics and optical sensor technologies, which supplies critical components such as photomultiplier tubes and silicon photomultipliers for DOT systems. Artinis Medical Systems is notable for its development of wearable and portable near-infrared spectroscopy (NIRS) and DOT devices, with a focus on brain and muscle imaging. Their systems are widely used in both clinical research and emerging point-of-care applications. Hitachi has also been active in the field, leveraging its expertise in medical imaging to develop multi-channel DOT systems for functional neuroimaging, particularly in pediatric and cognitive neuroscience settings.
Recent innovations center on miniaturization, higher channel counts, and improved spatial resolution. The integration of time-domain and frequency-domain techniques, as seen in products from Hamamatsu Photonics and Artinis Medical Systems, enables more accurate quantification of tissue optical properties and deeper tissue penetration. Advances in light sources, such as high-power LEDs and laser diodes, combined with sensitive detectors, are pushing the boundaries of what DOT can achieve in terms of depth and resolution.
Artificial intelligence and machine learning are increasingly being incorporated into DOT systems for real-time image reconstruction and artifact reduction, a trend expected to accelerate through 2025 and beyond. This is facilitating the transition of DOT from research laboratories to clinical environments, where rapid and reliable imaging is essential. Furthermore, the development of wearable DOT systems is opening new avenues for continuous monitoring in naturalistic settings, such as bedside monitoring in neonatal intensive care units or ambulatory brain function assessment.
Looking ahead, the DOT market is poised for further growth as regulatory approvals expand and clinical validation studies mature. Collaborations between device manufacturers, academic institutions, and healthcare providers are expected to drive the adoption of DOT in mainstream medical diagnostics, with a particular emphasis on neurology, oncology, and critical care. The next few years will likely see DOT systems becoming more compact, user-friendly, and integrated with other imaging modalities, solidifying their role in the future of non-invasive medical imaging.
Competitive Analysis: Leading Companies and Strategic Moves
The competitive landscape for Diffuse Optical Tomography (DOT) systems in 2025 is characterized by a mix of established medical device manufacturers, specialized optical imaging firms, and emerging technology startups. The sector is witnessing increased investment in research and development, strategic partnerships, and product innovation, as companies seek to address the growing demand for non-invasive, real-time imaging solutions in neurology, oncology, and functional brain imaging.
Among the leading players, Hamamatsu Photonics stands out for its advanced photonic components and integrated systems, which are widely used in DOT devices. The company continues to expand its portfolio with high-sensitivity detectors and light sources, supporting both OEMs and end-users in clinical and research settings. Hamamatsu Photonics is also actively collaborating with academic institutions to refine DOT technology for improved spatial resolution and depth penetration.
Another key competitor, Hitachi, Ltd., leverages its expertise in medical imaging and optoelectronics to offer DOT and related near-infrared spectroscopy (NIRS) systems. The company’s systems are recognized for their reliability and integration with multimodal imaging platforms, making them popular in both hospital and research environments. Hitachi, Ltd. is focusing on expanding its global footprint through partnerships with healthcare providers and research consortia, particularly in Europe and North America.
In the United States, Artinis Medical Systems is a prominent supplier of portable and wearable DOT and NIRS devices. The company’s emphasis on user-friendly, wireless systems has positioned it as a preferred choice for cognitive neuroscience and sports medicine applications. Artinis Medical Systems is investing in miniaturization and cloud-based data analytics, aiming to enhance the accessibility and scalability of DOT technology.
Emerging players such as NeuroLight Technologies (if confirmed operational) are entering the market with novel approaches, including AI-driven image reconstruction and real-time monitoring capabilities. These innovations are expected to drive competition and accelerate the adoption of DOT in new clinical and research domains.
Looking ahead, the competitive dynamics are likely to intensify as companies pursue regulatory approvals, expand into untapped markets, and integrate DOT with other imaging modalities. Strategic moves such as mergers, acquisitions, and cross-industry collaborations are anticipated, with a focus on improving system performance, reducing costs, and broadening clinical indications. The next few years will be pivotal as DOT systems transition from primarily research tools to mainstream clinical diagnostics, reshaping the landscape of non-invasive medical imaging.
Clinical Applications and Expanding Use Cases
Diffuse Optical Tomography (DOT) systems are increasingly being integrated into clinical workflows, with 2025 marking a period of notable expansion in both established and emerging medical applications. Traditionally, DOT has been utilized for functional brain imaging, breast cancer detection, and neonatal cerebral monitoring. However, recent technological advancements and validation studies are driving broader adoption and new clinical use cases.
In neuroimaging, DOT systems are gaining traction as a non-invasive, portable alternative to functional MRI, particularly for bedside monitoring in intensive care units and intraoperative settings. Companies such as Hamamatsu Photonics and Hitachi are at the forefront, offering advanced near-infrared spectroscopy (NIRS) and DOT platforms that enable real-time monitoring of cerebral oxygenation and hemodynamics. These systems are being increasingly adopted in pediatric neurology and stroke care, where rapid, continuous assessment is critical.
Breast imaging remains a key clinical domain for DOT, with systems designed to complement or, in some cases, provide alternatives to mammography, especially for women with dense breast tissue. Siemens Healthineers and Philips are actively developing and refining DOT-based breast imaging solutions, focusing on improving sensitivity and specificity for early cancer detection. Clinical trials underway in 2025 are expected to provide further evidence supporting DOT’s role in routine breast cancer screening and monitoring therapeutic response.
Emerging use cases are also being explored. In musculoskeletal medicine, DOT is being piloted for the assessment of muscle oxygenation and vascular health, with potential applications in sports medicine and rehabilitation. Additionally, the integration of DOT with other imaging modalities, such as ultrasound and MRI, is a growing trend, aiming to provide multi-parametric data for comprehensive diagnostics. Companies like NeuroMetrix are investigating hybrid systems that leverage DOT’s strengths in functional imaging.
Looking ahead, the clinical adoption of DOT systems is expected to accelerate, driven by ongoing miniaturization, improved data analytics, and increasing regulatory approvals. The next few years will likely see DOT incorporated into point-of-care devices and wearable platforms, expanding its reach into outpatient and home monitoring scenarios. As clinical evidence accumulates and technology matures, DOT is poised to become a standard tool in personalized and precision medicine across multiple specialties.
Regulatory Environment and Industry Standards
The regulatory environment for Diffuse Optical Tomography (DOT) systems is evolving rapidly as these devices gain traction in clinical and research settings. In 2025, DOT systems—used for non-invasive imaging of tissue hemodynamics and function—are subject to increasingly stringent oversight, particularly in major markets such as the United States, European Union, and Asia-Pacific. Regulatory agencies are focusing on both device safety and efficacy, as well as interoperability and data security, reflecting the growing integration of DOT with digital health platforms.
In the United States, DOT systems are regulated as medical devices by the Food and Drug Administration (FDA). Most DOT systems fall under Class II (moderate risk) or, in some cases, Class III (high risk) categories, depending on their intended use (diagnostic vs. research). The FDA requires premarket notification (510(k)) or premarket approval (PMA) for new DOT devices, with a strong emphasis on clinical validation and biocompatibility. Companies such as Artinis Medical Systems and Hamamatsu Photonics have successfully navigated these pathways for their DOT and near-infrared spectroscopy (NIRS) systems, setting precedents for future entrants.
In the European Union, DOT systems are governed by the Medical Device Regulation (MDR 2017/745), which came fully into effect in 2021 and continues to shape the regulatory landscape in 2025. The MDR imposes rigorous requirements for clinical evidence, post-market surveillance, and traceability. Notified Bodies play a central role in certifying DOT systems for CE marking. Companies like NIRx Medical Technologies and Brain Vision have adapted their quality management systems to comply with MDR, ensuring continued market access.
Industry standards are also maturing. The International Electrotechnical Commission (IEC) and International Organization for Standardization (ISO) are developing and updating standards relevant to DOT, such as IEC 60601 for electrical safety and ISO 13485 for quality management. In parallel, industry consortia and working groups are addressing interoperability and data format standards, which are crucial for integrating DOT data with hospital information systems and electronic health records.
Looking ahead, regulatory agencies are expected to further harmonize requirements for DOT systems, particularly regarding software as a medical device (SaMD) and artificial intelligence (AI) integration. The next few years will likely see increased collaboration between manufacturers, regulators, and standards bodies to streamline approval processes and foster innovation, while maintaining patient safety and data integrity.
Regional Market Dynamics: North America, Europe, Asia-Pacific, and Beyond
The global market for Diffuse Optical Tomography (DOT) systems is experiencing dynamic regional shifts, with North America, Europe, and Asia-Pacific emerging as key centers of innovation, adoption, and commercialization. As of 2025, North America remains at the forefront, driven by robust research funding, a strong presence of leading medical device manufacturers, and a well-established healthcare infrastructure. The United States, in particular, is home to several pioneering companies and academic institutions advancing DOT technology for applications in neurology, oncology, and neonatal care. Notable industry players such as Artinis Medical Systems and Hamamatsu Photonics have established significant footprints in the region, supporting both clinical and research deployments.
Europe continues to demonstrate strong momentum, underpinned by collaborative research initiatives and supportive regulatory frameworks. Countries like Germany, the United Kingdom, and the Netherlands are investing in translational research to bring DOT systems from laboratory settings to clinical practice. European companies, including Artinis Medical Systems (Netherlands) and NeuroMetrix (UK), are actively developing portable and wearable DOT solutions, targeting both hospital and point-of-care environments. The region’s emphasis on non-invasive imaging and personalized medicine is expected to further accelerate DOT adoption in the coming years.
The Asia-Pacific region is rapidly emerging as a significant growth market, fueled by expanding healthcare infrastructure, increasing investments in medical technology, and a rising prevalence of neurological and oncological disorders. Japan, China, and South Korea are leading the charge, with local manufacturers such as Hamamatsu Photonics (Japan) and Hitachi (Japan) investing in advanced DOT platforms. These companies are focusing on miniaturization, cost reduction, and integration with other imaging modalities to cater to diverse clinical needs and resource-constrained settings.
Beyond these core regions, emerging markets in Latin America and the Middle East are beginning to explore DOT technologies, primarily through academic collaborations and pilot projects. However, widespread adoption in these areas is currently limited by infrastructure and regulatory challenges.
Looking ahead, the next few years are expected to see intensified competition and cross-regional partnerships, as manufacturers seek to expand their global reach and address unmet clinical needs. Advances in photonics, data analytics, and wearable device design are likely to drive further innovation, making DOT systems more accessible and versatile across diverse healthcare settings worldwide.
Investment, M&A, and Funding Trends
Investment and funding activity in the diffuse optical tomography (DOT) systems sector has accelerated into 2025, reflecting the growing clinical and research adoption of non-invasive optical imaging. The sector is characterized by a mix of established medical device manufacturers, university spinouts, and emerging startups, each attracting capital to advance hardware miniaturization, software integration, and clinical validation.
Key players such as Hamamatsu Photonics and Hitachi continue to invest in R&D for next-generation DOT platforms, leveraging their expertise in photonics and medical imaging. Hamamatsu Photonics has expanded its optical sensor manufacturing capacity, signaling confidence in the growing demand for DOT components. Meanwhile, Hitachi has maintained its commitment to optical topography systems, with ongoing collaborations with academic medical centers to validate new clinical applications.
On the startup front, companies such as NeuroMetrix and Artinis Medical Systems have secured new funding rounds in the past year, aimed at scaling production and expanding their product portfolios. Artinis Medical Systems, for example, has focused on wearable DOT devices for brain monitoring, attracting both venture capital and strategic investment from larger medtech firms. These investments are often tied to milestones in regulatory approval and clinical trial progress, with investors seeking to capitalize on the growing use of DOT in neurology, oncology, and rehabilitation.
Mergers and acquisitions have also shaped the landscape. Larger imaging companies are increasingly acquiring or partnering with DOT technology developers to complement their existing product lines. This trend is exemplified by recent partnerships between established imaging firms and DOT innovators, aiming to integrate optical tomography with MRI or ultrasound platforms for multi-modal imaging solutions.
Looking ahead, the outlook for investment and M&A in DOT systems remains robust. The sector is expected to benefit from increased healthcare digitization, the push for portable and point-of-care diagnostics, and the expansion of optical imaging into new therapeutic areas. As clinical evidence supporting DOT’s efficacy grows, further rounds of funding and strategic acquisitions are anticipated, particularly as companies seek to address unmet needs in brain health, cancer detection, and pediatric care.
Challenges, Barriers, and Risk Factors
Diffuse Optical Tomography (DOT) systems, which utilize near-infrared light to generate functional images of tissue, are gaining traction in clinical and research settings. However, several challenges, barriers, and risk factors continue to shape the landscape of DOT adoption and development as of 2025 and for the foreseeable future.
A primary technical challenge remains the limited spatial resolution of DOT compared to established imaging modalities such as MRI or CT. The inherent scattering of photons in biological tissue restricts the achievable image clarity, particularly for deep tissue imaging. While advances in hardware and reconstruction algorithms are ongoing, companies such as Hamamatsu Photonics and Artinis Medical Systems are actively working to improve detector sensitivity and signal processing, but the gap in resolution persists.
Another significant barrier is the complexity of system calibration and the need for robust, user-friendly software. Many DOT systems require careful calibration and expertise to operate, which can limit their adoption in routine clinical workflows. Efforts by manufacturers like NeuroLight and Gowerlabs are focused on developing more automated and intuitive interfaces, yet widespread standardization is still lacking.
Regulatory hurdles also present a risk factor for DOT system developers. Gaining approval from regulatory bodies such as the FDA or EMA requires extensive clinical validation, which can be time-consuming and costly. As of 2025, only a limited number of DOT systems have received such clearances for specific clinical applications, and most are still used primarily in research settings. This regulatory uncertainty can deter investment and slow market growth.
Cost remains a barrier, particularly for advanced multi-channel or high-density DOT systems. The price of these systems, which can include custom optoelectronic components and sophisticated software, may be prohibitive for smaller clinics or research groups. Companies like NIRx Medical Technologies and Hidex are working to offer scalable solutions, but affordability is still a concern.
Finally, there are risks related to data interpretation and clinical utility. The translation of DOT data into actionable clinical information is not always straightforward, and there is a need for more large-scale, multi-center studies to validate its diagnostic and prognostic value. Without clear clinical guidelines and evidence, adoption may remain limited.
Looking ahead, overcoming these challenges will require continued collaboration between manufacturers, clinicians, and regulatory agencies. Advances in photonics, machine learning, and system integration are expected to gradually address many of these barriers, but significant work remains to ensure DOT systems can achieve their full potential in mainstream healthcare.
Future Outlook: Opportunities and Strategic Recommendations
Diffuse Optical Tomography (DOT) systems are poised for significant advancements and market expansion in 2025 and the coming years, driven by technological innovation, clinical adoption, and strategic industry initiatives. The increasing demand for non-invasive, real-time imaging solutions in neurology, oncology, and neonatal care is catalyzing both research and commercial interest. Key players are investing in next-generation hardware and software, aiming to improve spatial resolution, portability, and user-friendliness.
A major opportunity lies in the integration of DOT with other imaging modalities, such as MRI and ultrasound, to provide complementary functional and anatomical information. Companies like Hamamatsu Photonics, a global leader in photonics and optical sensor technologies, are actively developing advanced photodetectors and light sources that enhance DOT system sensitivity and accuracy. Similarly, Artinis Medical Systems is focusing on wearable and wireless DOT solutions, targeting both clinical and research applications, which aligns with the broader trend toward point-of-care and home-based monitoring.
Strategic collaborations between device manufacturers, academic institutions, and healthcare providers are expected to accelerate clinical validation and regulatory approvals. For instance, Neurolight and NIRx Medical Technologies are expanding their partnerships to facilitate multi-center trials and broaden the clinical evidence base for DOT in brain imaging and functional monitoring. These efforts are crucial for establishing DOT as a standard tool in neurocritical care and cognitive neuroscience.
Artificial intelligence and machine learning are anticipated to play a transformative role in DOT data analysis, enabling automated image reconstruction and interpretation. This will not only improve diagnostic accuracy but also reduce the learning curve for clinicians. Companies such as Artinis Medical Systems and Hamamatsu Photonics are investing in software platforms that leverage AI for real-time data processing and visualization.
Looking ahead, the DOT market is expected to benefit from favorable reimbursement policies and increased funding for non-invasive imaging research. However, challenges remain, including the need for standardized protocols, interoperability with hospital IT systems, and cost-effective manufacturing. Strategic recommendations for stakeholders include prioritizing user-centric design, fostering cross-sector partnerships, and investing in education to drive adoption among clinicians and researchers. As the technology matures, DOT systems are well-positioned to become integral components of precision medicine and personalized healthcare.



