Swept-Source Optical Coherence Tomography Systems in 2025: Unveiling the Future of High-Resolution Imaging and Market Expansion. Explore How Advanced SS-OCT Technologies Are Shaping Diagnostics and Research.
- Executive Summary: Key Trends and Market Drivers in 2025
- Technology Overview: Principles and Innovations in Swept-Source OCT
- Competitive Landscape: Leading Manufacturers and Strategic Partnerships
- Market Segmentation: Applications in Ophthalmology, Cardiology, and Beyond
- Regional Analysis: Growth Hotspots and Emerging Markets
- Regulatory Environment and Industry Standards
- Recent Product Launches and R&D Breakthroughs
- Market Forecast 2025–2030: Revenue, Volume, and Adoption Rates
- Challenges and Barriers: Technical, Clinical, and Commercial Hurdles
- Future Outlook: Next-Generation SS-OCT and Long-Term Opportunities
- Sources & References
Executive Summary: Key Trends and Market Drivers in 2025
Swept-Source Optical Coherence Tomography (SS-OCT) systems are poised for significant growth and technological advancement in 2025, driven by increasing clinical adoption, ongoing innovation, and expanding applications beyond ophthalmology. SS-OCT leverages rapidly tunable lasers to achieve deeper tissue penetration and faster imaging speeds compared to traditional spectral-domain OCT, making it a preferred modality for high-resolution, volumetric imaging.
A key trend in 2025 is the integration of SS-OCT into multimodal diagnostic platforms, particularly in ophthalmology, where it is used for detailed visualization of the retina, choroid, and anterior segment. Leading manufacturers such as Topcon Corporation and Carl Zeiss Meditec AG continue to expand their SS-OCT product portfolios, focusing on enhanced imaging speed, improved user interfaces, and artificial intelligence (AI)-driven analytics. These advancements are enabling earlier detection and more precise monitoring of diseases such as age-related macular degeneration and diabetic retinopathy.
Another driver is the growing demand for non-invasive, real-time imaging in cardiology, dermatology, and oncology. Companies like Canon Inc. are actively developing SS-OCT systems for broader clinical and research applications, leveraging their expertise in optics and imaging. The miniaturization of swept-source lasers and the reduction in system costs are making SS-OCT more accessible to a wider range of healthcare providers, including outpatient clinics and research institutions.
Regulatory approvals and reimbursement policies are also shaping the market landscape. In 2025, more SS-OCT systems are expected to receive clearances from regulatory bodies in North America, Europe, and Asia, facilitating wider adoption. Industry organizations such as the American Academy of Ophthalmology are actively promoting the clinical benefits of advanced OCT technologies, further driving demand.
Looking ahead, the outlook for SS-OCT systems remains robust. The convergence of AI, cloud-based data management, and telemedicine is expected to further enhance the utility and reach of SS-OCT, particularly in remote and underserved regions. Strategic collaborations between device manufacturers, software developers, and healthcare providers are anticipated to accelerate innovation and streamline clinical workflows. As a result, SS-OCT is set to play an increasingly central role in precision diagnostics and personalized medicine over the next several years.
Technology Overview: Principles and Innovations in Swept-Source OCT
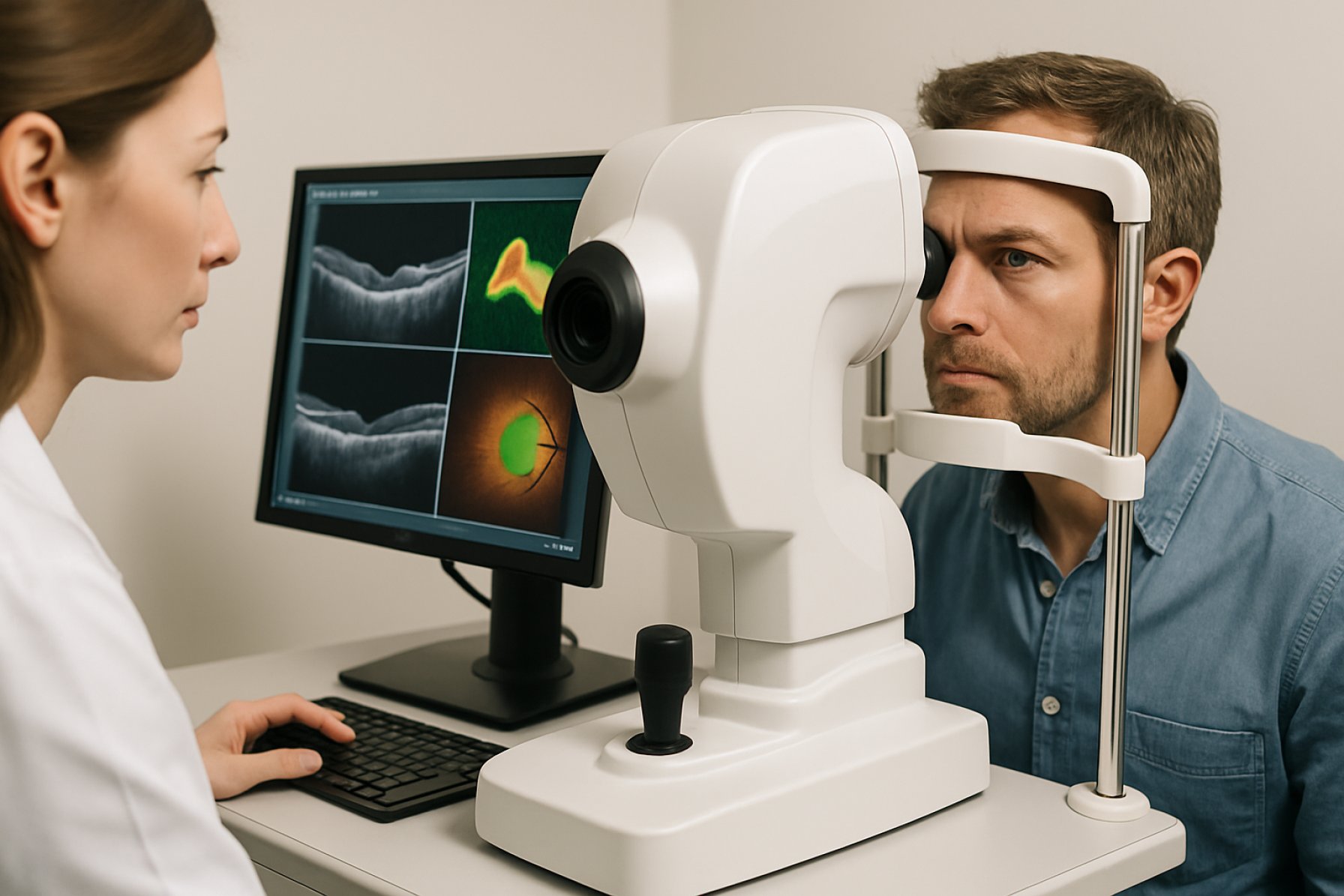
Swept-Source Optical Coherence Tomography (SS-OCT) represents a significant advancement in the field of high-resolution, non-invasive imaging, particularly for ophthalmology and biomedical research. The core principle of SS-OCT involves the use of a rapidly tunable laser (swept-source) that scans across a range of wavelengths, enabling the acquisition of depth-resolved images with high speed and sensitivity. Unlike traditional spectral-domain OCT, SS-OCT systems offer deeper tissue penetration, reduced sensitivity roll-off, and faster imaging speeds, making them highly suitable for detailed visualization of ocular structures and other biological tissues.
As of 2025, SS-OCT technology continues to evolve, driven by innovations in tunable laser sources, photodetectors, and signal processing algorithms. Leading manufacturers such as Topcon Corporation, Canon Inc., and Carl Zeiss AG have integrated SS-OCT into their flagship ophthalmic imaging platforms. These systems leverage vertical-cavity surface-emitting lasers (VCSELs) and other advanced swept-source technologies to achieve imaging speeds exceeding 100,000 A-scans per second, with axial resolutions in the range of 5–8 microns. Such performance enables comprehensive visualization of the retina, choroid, and anterior segment, supporting both clinical diagnostics and research applications.
Recent years have seen the introduction of multimodal platforms that combine SS-OCT with complementary imaging modalities, such as OCT angiography (OCTA), to provide detailed vascular mapping without the need for contrast agents. Companies like NIDEK Co., Ltd. and Huvitz Co., Ltd. are actively developing and commercializing such integrated systems, expanding the clinical utility of SS-OCT in the diagnosis and management of retinal diseases, glaucoma, and anterior segment disorders.
On the component level, suppliers such as Santec Corporation and Lumentum Holdings Inc. are advancing the performance of swept-source lasers, focusing on broader tuning ranges, higher output power, and improved wavelength stability. These innovations are expected to further enhance image quality and enable new applications in fields such as cardiology, dermatology, and industrial inspection.
Looking ahead to the next few years, the SS-OCT sector is poised for continued growth, with ongoing research into miniaturization, cost reduction, and artificial intelligence-driven image analysis. The integration of SS-OCT into portable and point-of-care devices is anticipated, broadening access to advanced imaging in diverse clinical settings. As manufacturers and component suppliers continue to push the boundaries of speed, resolution, and versatility, SS-OCT is set to remain at the forefront of optical imaging innovation through 2025 and beyond.
Competitive Landscape: Leading Manufacturers and Strategic Partnerships
The competitive landscape for Swept-Source Optical Coherence Tomography (SS-OCT) systems in 2025 is characterized by a dynamic interplay among established medical device manufacturers, innovative startups, and strategic partnerships aimed at advancing imaging performance and expanding clinical applications. The market is led by a handful of global players with robust R&D capabilities, extensive distribution networks, and a strong focus on ophthalmology, cardiology, and industrial inspection.
Among the most prominent manufacturers, Canon Inc. continues to be a key innovator, leveraging its deep expertise in imaging technologies. Canon’s acquisition of Toshiba Medical Systems has further strengthened its position in the ophthalmic imaging sector, with the company’s swept-source OCT platforms widely adopted in clinical practice. Topcon Corporation is another major player, recognized for its DRI OCT Triton series, which utilizes swept-source technology to deliver high-speed, deep-penetration imaging for retinal and choroidal assessment. Topcon’s global reach and ongoing investment in AI integration are expected to sustain its leadership through 2025 and beyond.
Carl Zeiss Meditec AG remains a formidable competitor, with its PLEX Elite 9000 system setting benchmarks in high-resolution, widefield imaging. Zeiss’s strategic collaborations with research institutions and clinical partners are accelerating the development of next-generation SS-OCT solutions, particularly for early detection of retinal diseases. NIDEK CO., LTD. also maintains a significant presence, focusing on user-friendly, versatile SS-OCT platforms that appeal to both large hospitals and smaller clinics.
In the United States, Optovue, Inc. (now part of Lumentum Holdings Inc.) has been instrumental in commercializing swept-source OCT angiography, with its AngioVue platform widely recognized for non-invasive vascular imaging. The integration of Optovue’s technology into Lumentum’s broader photonics portfolio is expected to drive further innovation and market expansion.
Strategic partnerships are increasingly shaping the competitive landscape. Collaborations between device manufacturers and academic centers are fostering rapid prototyping and clinical validation of novel SS-OCT applications, such as intraoperative imaging and neuro-ophthalmology. Additionally, alliances with software companies are enabling the integration of advanced analytics and AI-driven diagnostic tools, enhancing the clinical utility of SS-OCT systems.
Looking ahead, the competitive environment is likely to intensify as new entrants, particularly from Asia, introduce cost-effective swept-source platforms. However, established players with strong intellectual property portfolios, regulatory expertise, and global service networks are expected to maintain a competitive edge through continuous innovation and strategic alliances.
Market Segmentation: Applications in Ophthalmology, Cardiology, and Beyond
Swept-Source Optical Coherence Tomography (SS-OCT) systems have become pivotal in advancing diagnostic imaging across multiple medical fields, with ophthalmology and cardiology representing the largest application segments as of 2025. The technology’s ability to deliver high-speed, deep-penetration, and high-resolution imaging has driven its adoption in both established and emerging clinical domains.
In ophthalmology, SS-OCT has rapidly become the gold standard for retinal and anterior segment imaging. Its longer wavelength light sources enable superior visualization of deeper ocular structures, such as the choroid and sclera, compared to earlier spectral-domain systems. Leading manufacturers like Topcon Corporation and Canon Inc. have developed advanced SS-OCT platforms, such as the DRI OCT Triton and Xephilio OCT-S1, which are widely adopted in eye clinics and research centers globally. These systems support early detection and monitoring of diseases like age-related macular degeneration, diabetic retinopathy, and glaucoma, and are increasingly integrated with artificial intelligence for automated analysis.
Cardiology represents another significant and growing segment for SS-OCT. The technology is used for intravascular imaging, providing detailed cross-sectional views of coronary arteries to guide interventions such as stent placement. Companies like Abbott have commercialized SS-OCT-based intravascular imaging systems, which offer faster acquisition speeds and improved tissue penetration compared to time-domain OCT. This enables more accurate assessment of plaque morphology and stent apposition, supporting better clinical outcomes in percutaneous coronary interventions.
Beyond ophthalmology and cardiology, SS-OCT is expanding into additional medical and industrial applications. In dermatology, it is being explored for non-invasive skin cancer diagnostics and monitoring of treatment response. In dentistry, SS-OCT systems are under evaluation for imaging dental tissues and detecting caries. The technology is also being adapted for use in endoscopy, enabling real-time, high-resolution imaging of gastrointestinal and respiratory tract tissues. Companies such as NIDEK Co., Ltd. and Tomey Corporation are actively developing SS-OCT solutions for these broader applications.
Looking ahead, the SS-OCT market is expected to see continued growth through 2025 and beyond, driven by ongoing technological innovation, expanding clinical indications, and increasing integration with digital health platforms. As manufacturers invest in miniaturization, faster scanning speeds, and AI-powered analytics, SS-OCT systems are poised to become even more versatile tools across a widening array of medical specialties.
Regional Analysis: Growth Hotspots and Emerging Markets
Swept-Source Optical Coherence Tomography (SS-OCT) systems are experiencing dynamic regional growth, with several geographic hotspots and emerging markets shaping the sector’s outlook for 2025 and beyond. The technology’s adoption is closely tied to the expansion of ophthalmic care infrastructure, increasing prevalence of retinal diseases, and the push for advanced diagnostic capabilities in both developed and developing economies.
In North America, the United States remains the largest market for SS-OCT systems, driven by robust healthcare spending, a high concentration of leading ophthalmic device manufacturers, and a strong clinical research ecosystem. Companies such as Carl Zeiss Meditec and Topcon Corporation maintain significant market presence, with ongoing product launches and clinical collaborations. The region’s growth is further supported by favorable reimbursement policies and the rapid integration of SS-OCT into routine ophthalmic practice.
Europe is another established market, with countries like Germany, the United Kingdom, and France at the forefront. The region benefits from a well-developed healthcare infrastructure and a high rate of adoption of advanced imaging technologies. Canon Inc. and NIDEK CO., LTD. are notable players, leveraging their strong distribution networks and partnerships with academic institutions to expand their SS-OCT offerings. The European market is also characterized by a growing emphasis on early detection of age-related macular degeneration and diabetic retinopathy, further fueling demand.
Asia-Pacific is emerging as the fastest-growing region for SS-OCT systems, with significant momentum in Japan, China, South Korea, and India. Japan, home to major manufacturers like Topcon Corporation and Canon Inc., leads in both innovation and adoption. China’s market is expanding rapidly, propelled by government initiatives to modernize healthcare and a rising middle class seeking advanced eye care. Local manufacturers are increasingly entering the field, intensifying competition and driving down costs. India, while still in the early stages, is witnessing increased investment in ophthalmic clinics and diagnostic centers, suggesting strong future growth potential.
Looking ahead to the next few years, Latin America and the Middle East are expected to see gradual uptake of SS-OCT systems, primarily in urban centers and private healthcare facilities. While these regions currently face challenges such as limited access and budget constraints, ongoing healthcare reforms and international partnerships are likely to improve market penetration.
Overall, the global SS-OCT market in 2025 is marked by strong growth in Asia-Pacific, steady expansion in North America and Europe, and emerging opportunities in other regions. The competitive landscape is shaped by established multinational manufacturers and a growing cohort of regional players, all vying to address the increasing demand for high-resolution, non-invasive ocular imaging.
Regulatory Environment and Industry Standards
The regulatory environment for Swept-Source Optical Coherence Tomography (SS-OCT) systems is evolving rapidly as these devices become increasingly integral to ophthalmic diagnostics and other biomedical imaging applications. In 2025, regulatory agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) continue to play pivotal roles in shaping the approval pathways and post-market surveillance requirements for SS-OCT systems.
SS-OCT systems are classified as Class II or Class IIa medical devices in most jurisdictions, necessitating rigorous premarket submissions that include clinical data, safety, and performance evidence. The FDA’s 510(k) clearance process remains the primary route for market entry in the United States, with recent clearances granted to advanced SS-OCT platforms from leading manufacturers such as Carl Zeiss Meditec, Topcon Corporation, and Canon Inc.. These companies have demonstrated compliance with standards such as IEC 60601 for electrical safety and ISO 13485 for quality management systems, which are increasingly harmonized across major markets.
In the European Union, the transition from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) has introduced more stringent requirements for clinical evaluation and post-market surveillance. SS-OCT manufacturers are now required to provide more comprehensive clinical data and implement robust post-market follow-up plans. Companies like NIDEK Co., Ltd. and Haag-Streit AG have adapted their regulatory strategies to align with MDR, ensuring continued CE marking for their SS-OCT systems.
Industry standards are also advancing, with organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) updating relevant standards to address the unique characteristics of SS-OCT technology. The adoption of standards like ISO 10993 for biocompatibility and IEC 62304 for medical device software lifecycle processes is becoming more widespread among manufacturers.
Looking ahead, regulatory bodies are expected to place greater emphasis on cybersecurity, interoperability, and artificial intelligence (AI) integration in SS-OCT systems. Manufacturers including Optovue, Inc. (now part of Lumentum Holdings Inc.) are actively engaging with regulators to ensure that new features, such as AI-driven image analysis, meet evolving safety and efficacy standards. The next few years will likely see further harmonization of global regulatory requirements, facilitating faster market access and broader adoption of SS-OCT technologies worldwide.
Recent Product Launches and R&D Breakthroughs
The landscape of Swept-Source Optical Coherence Tomography (SS-OCT) systems is experiencing significant momentum in 2025, driven by both established manufacturers and innovative entrants. SS-OCT, which leverages rapidly tunable lasers for deeper tissue penetration and faster imaging, is increasingly favored in ophthalmology, cardiology, and industrial inspection.
A major player, Topcon Corporation, continues to expand its DRI OCT Triton series, which utilizes a 1,050 nm wavelength swept-source laser. In 2024 and early 2025, Topcon has focused on enhancing image acquisition speed and integrating artificial intelligence (AI) for automated retinal layer segmentation, aiming to streamline clinical workflows and improve diagnostic accuracy. The company’s global distribution and service network further solidify its position as a leader in ophthalmic SS-OCT.
Another key innovator, Carl Zeiss Meditec AG, has advanced its PLEX Elite 9000 platform, which is widely adopted in research and high-end clinical settings. In 2025, Zeiss is emphasizing multimodal imaging capabilities, combining SS-OCT with angiography and adaptive optics. This integration allows for unprecedented visualization of microvascular structures and cellular-level changes, supporting both clinical diagnostics and longitudinal research studies.
In the United States, Canon Inc. has introduced new SS-OCT models under its Xephilio brand, focusing on compact form factors and user-friendly interfaces. Canon’s 2025 releases highlight improved scanning speeds and enhanced software for real-time 3D visualization, targeting both large hospitals and smaller clinics seeking to upgrade from spectral-domain OCT systems.
On the R&D front, Santec Corporation and Laser Components GmbH are pushing the boundaries of swept-source laser technology. Santec, a leading supplier of tunable lasers, has announced new high-speed, wide-sweep laser modules in 2025, enabling faster volumetric imaging and deeper tissue penetration. Laser Components is focusing on miniaturization and integration, aiming to facilitate the development of portable and point-of-care SS-OCT devices.
Looking ahead, the next few years are expected to see further convergence of SS-OCT with AI-driven analytics, cloud-based data management, and telemedicine platforms. Industry leaders are investing in interoperability and open software ecosystems, anticipating broader adoption in both clinical and industrial markets. As regulatory approvals for new indications expand, SS-OCT systems are poised to become even more central to precision diagnostics and monitoring across multiple disciplines.
Market Forecast 2025–2030: Revenue, Volume, and Adoption Rates
The market for Swept-Source Optical Coherence Tomography (SS-OCT) systems is poised for robust growth between 2025 and 2030, driven by technological advancements, expanding clinical applications, and increasing adoption in ophthalmology and beyond. SS-OCT systems, which utilize rapidly tunable lasers to achieve deeper tissue penetration and faster imaging speeds compared to traditional spectral-domain OCT, are increasingly favored in both research and clinical settings.
Revenue projections for the global SS-OCT market indicate a compound annual growth rate (CAGR) in the high single digits through 2030. This growth is underpinned by rising demand for advanced diagnostic imaging in ophthalmology, particularly for retinal and choroidal imaging, as well as emerging applications in cardiology, dermatology, and industrial inspection. Leading manufacturers such as Topcon Corporation, Carl Zeiss Meditec AG, and Canon Inc. are expected to maintain significant market shares, leveraging their established distribution networks and ongoing investments in R&D.
In terms of volume, the annual shipment of SS-OCT systems is projected to increase steadily, with ophthalmic clinics and hospitals in North America, Europe, and Asia-Pacific driving the bulk of demand. The adoption rate of SS-OCT is anticipated to accelerate as device costs gradually decrease and reimbursement policies become more favorable, particularly in developed healthcare markets. The introduction of compact, user-friendly, and AI-integrated SS-OCT platforms is expected to further broaden the customer base, enabling penetration into smaller practices and emerging markets.
Key industry players are actively expanding their SS-OCT portfolios. NIDEK Co., Ltd. and Huvitz Co., Ltd. are notable for their focus on cost-effective solutions, targeting mid-tier and value-conscious segments. Meanwhile, Leica Microsystems and Tomey Corporation are investing in high-resolution and multimodal imaging capabilities, aiming to differentiate their offerings in a competitive landscape.
- By 2025, SS-OCT systems are expected to account for a growing share of new OCT installations, with adoption rates surpassing 40% in major ophthalmic centers.
- Revenue from SS-OCT devices is forecasted to exceed several hundred million USD globally by 2030, with Asia-Pacific emerging as the fastest-growing regional market.
- Integration with telemedicine and AI-driven analytics is anticipated to further boost adoption, particularly in remote diagnostics and population health screening programs.
Overall, the outlook for SS-OCT systems from 2025 to 2030 is highly positive, with sustained innovation, expanding clinical utility, and increasing accessibility driving both revenue and volume growth across key global markets.
Challenges and Barriers: Technical, Clinical, and Commercial Hurdles
Swept-Source Optical Coherence Tomography (SS-OCT) systems have rapidly advanced, yet several technical, clinical, and commercial challenges persist as of 2025, shaping the trajectory of their adoption and development in the coming years.
Technical Barriers remain significant. SS-OCT relies on tunable lasers with high sweep speeds and broad bandwidths, which are complex and costly to manufacture. Achieving stable, long-wavelength sources (typically around 1050 nm for ophthalmic use) with low noise and high reliability is a persistent engineering challenge. Leading manufacturers such as Topcon Corporation and Canon Inc. have made strides in miniaturizing and stabilizing these light sources, but further improvements are needed to reduce system size, power consumption, and cost for broader clinical deployment. Additionally, the high data rates generated by SS-OCT require advanced processing hardware and software, which can limit integration into compact or portable devices.
Clinical Hurdles are also prominent. While SS-OCT offers deeper tissue penetration and faster imaging compared to spectral-domain OCT, translating these advantages into clear clinical outcomes is ongoing. There is a need for more large-scale, longitudinal studies to validate the clinical utility of SS-OCT in diagnosing and managing diseases beyond ophthalmology, such as cardiology and dermatology. Regulatory approval processes, particularly in the US and EU, can be lengthy due to the need for robust evidence of safety and efficacy. Companies like Carl Zeiss Meditec AG and NIDEK CO., LTD. are actively engaged in clinical collaborations to expand the evidence base, but widespread clinical adoption is still hampered by the lack of standardized protocols and reimbursement pathways.
Commercial Barriers include high initial investment costs for healthcare providers, which can be prohibitive for smaller clinics or emerging markets. The price of SS-OCT systems remains substantially higher than conventional OCT, limiting accessibility. Furthermore, the market is dominated by a few established players—such as Topcon Corporation, Canon Inc., and Carl Zeiss Meditec AG—which can stifle competition and innovation from smaller entrants. Service and maintenance requirements, as well as the need for specialized training, add to the total cost of ownership.
Looking ahead, overcoming these barriers will require continued innovation in laser technology, data processing, and clinical validation. Industry collaboration and partnerships with academic institutions are expected to play a key role in addressing these challenges and expanding the reach of SS-OCT systems in the next few years.
Future Outlook: Next-Generation SS-OCT and Long-Term Opportunities
Swept-Source Optical Coherence Tomography (SS-OCT) systems are poised for significant advancements in 2025 and the following years, driven by both technological innovation and expanding clinical applications. The next generation of SS-OCT is expected to deliver higher imaging speeds, deeper tissue penetration, and improved image resolution, enabling more precise diagnostics across ophthalmology, cardiology, and other medical fields.
Key industry leaders such as Topcon Corporation, Carl Zeiss Meditec AG, and Canon Inc. are actively investing in research and development to enhance SS-OCT platforms. For instance, Topcon’s DRI OCT Triton series and Zeiss’s PLEX Elite 9000 have set benchmarks in widefield and high-speed imaging, and both companies have signaled ongoing efforts to further increase scanning speeds and automate image analysis using artificial intelligence. Canon, with its Xephilio OCT-S1, is also focusing on expanding the clinical utility of SS-OCT by integrating multimodal imaging capabilities.
Emerging players and component suppliers, such as Santec Corporation and Lumentum Holdings Inc., are contributing to the evolution of SS-OCT by developing advanced tunable lasers and photonic components. These innovations are expected to reduce system costs and footprint, making SS-OCT more accessible for point-of-care and portable applications. The miniaturization of swept-source lasers and the integration of photonic integrated circuits are anticipated to be key trends shaping the market through 2025 and beyond.
In terms of clinical adoption, SS-OCT is increasingly recognized for its ability to visualize deeper ocular structures, such as the choroid and sclera, and for its utility in non-ophthalmic fields like dermatology and intravascular imaging. Regulatory approvals and reimbursement expansions in major markets are likely to accelerate adoption rates. Industry organizations, including the American Academy of Ophthalmology, continue to highlight the growing evidence base supporting SS-OCT’s diagnostic value, which is expected to drive further integration into routine clinical workflows.
Looking ahead, the convergence of SS-OCT with artificial intelligence, cloud-based data management, and telemedicine platforms is anticipated to unlock new long-term opportunities. These include remote diagnostics, population screening, and personalized disease monitoring. As the technology matures, SS-OCT systems are expected to become more compact, affordable, and versatile, broadening their impact across healthcare and research domains.
Sources & References
- Topcon Corporation
- Canon Inc.
- Carl Zeiss AG
- NIDEK Co., Ltd.
- Huvitz Co., Ltd.
- Santec Corporation
- Lumentum Holdings Inc.
- Lumentum Holdings Inc.
- Tomey Corporation
- Topcon Corporation
- Haag-Streit AG
- Laser Components GmbH
- Leica Microsystems
- Tomey Corporation



